Search
- Page Path
- HOME > Search
Original Articles
- Comparison of atezolizumab plus bevacizumab and lenvatinib for hepatocellular carcinoma with portal vein tumor thrombosis
- Jeayeon Park, Yun Bin Lee, Yunmi Ko, Youngsu Park, Hyunjae Shin, Moon Haeng Hur, Min Kyung Park, Dae-Won Lee, Eun Ju Cho, Kyung-Hun Lee, Jeong-Hoon Lee, Su Jong Yu, Tae-Yong Kim, Yoon Jun Kim, Tae-You Kim, Jung-Hwan Yoon
- J Liver Cancer. 2024;24(1):81-91. Published online January 19, 2024
- DOI: https://doi.org/10.17998/jlc.2023.12.25
- 1,062 Views
- 135 Downloads
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background/Aim
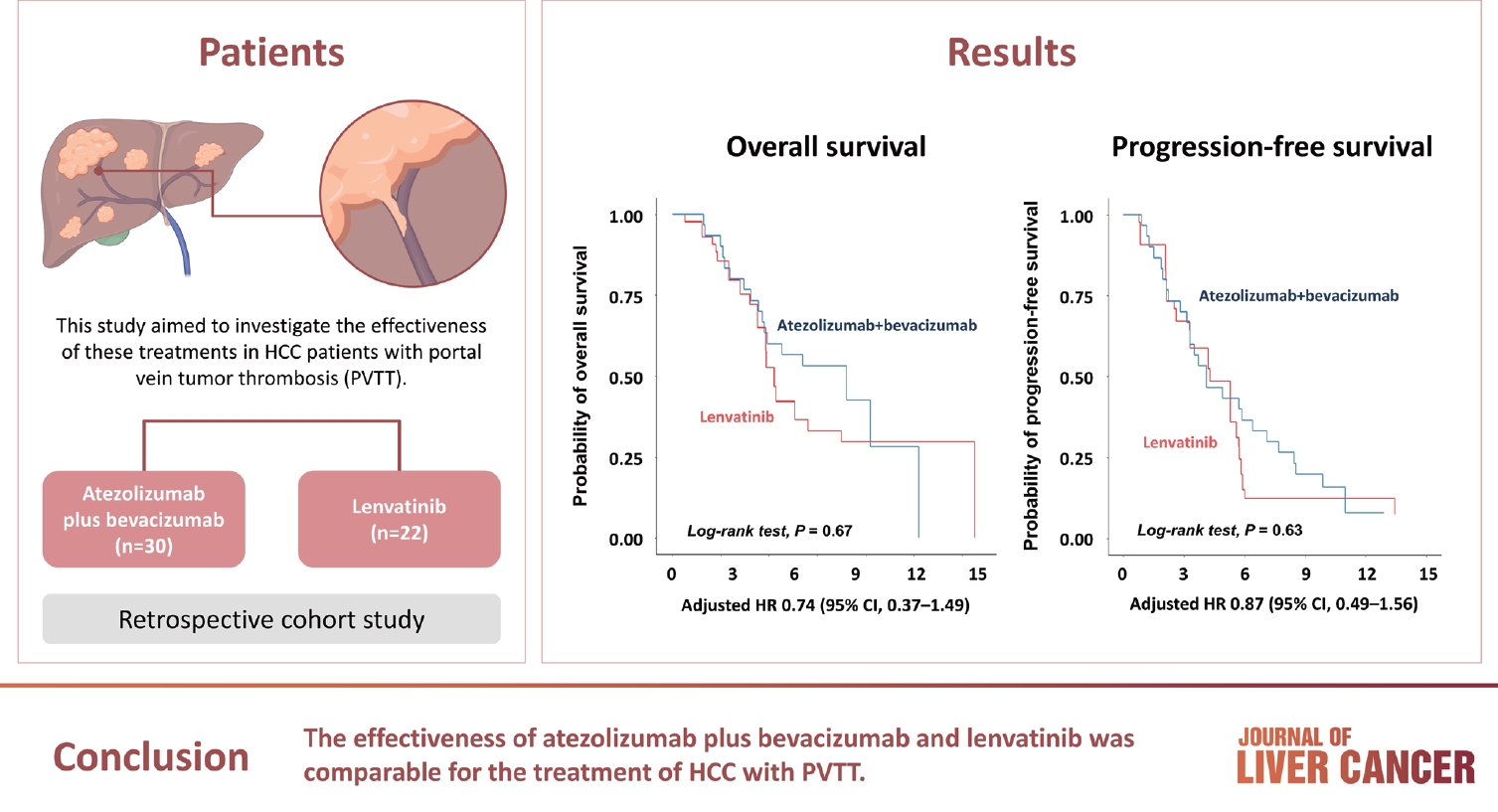
Atezolizumab plus bevacizumab and lenvatinib are currently available as first-line therapy for the treatment of unresectable hepatocellular carcinoma (HCC). However, comparative efficacy studies are still limited. This study aimed to investigate the effectiveness of these treatments in HCC patients with portal vein tumor thrombosis (PVTT).
Methods
We retrospectively included patients who received either atezolizumab plus bevacizumab or lenvatinib as first-line systemic therapy for HCC with PVTT. Primary endpoint was overall survival (OS), and secondary endpoints included progressionfree survival (PFS) and disease control rate (DCR) determined by response evaluation criteria in solid tumors, version 1.1.
Results
A total of 52 patients were included: 30 received atezolizumab plus bevacizumab and 22 received lenvatinib. The median follow-up duration was 6.4 months (interquartile range, 3.9-9.8). The median OS was 10.8 months (95% confidence interval [CI], 5.7 to not estimated) with atezolizumab plus bevacizumab and 5.8 months (95% CI, 4.8 to not estimated) with lenvatinib (P=0.26 by log-rank test). There was no statistically significant difference in OS (adjusted hazard ratio [aHR], 0.71; 95% CI, 0.34-1.49; P=0.37). The median PFS was similar (P=0.63 by log-rank test), with 4.1 months (95% CI, 3.3-7.7) for atezolizumab plus bevacizumab and 4.3 months (95% CI, 2.6-5.8) for lenvatinib (aHR, 0.93; 95% CI, 0.51-1.69; P=0.80). HRs were similar after inverse probability treatment weighting. The DCRs were 23.3% and 18.2% in patients receiving atezolizumab plus bevacizumab and lenvatinib, respectively (P=0.74).
Conclusion
The effectiveness of atezolizumab plus bevacizumab and lenvatinib was comparable for the treatment of HCC with PVTT.

- Use of doxorubicin-eluting bead transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein invasion: a prospective study
- Su Jong Yu, Yun Bin Lee, Eun Ju Cho, Jeong-Hoon Lee, Hyo-Cheol Kim, Jin Wook Chung, Jung-Hwan Yoon, Yoon Jun Kim
- J Liver Cancer. 2023;23(1):166-176. Published online March 3, 2023
- DOI: https://doi.org/10.17998/jlc.2023.02.08
- 2,106 Views
- 96 Downloads
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background/Aim
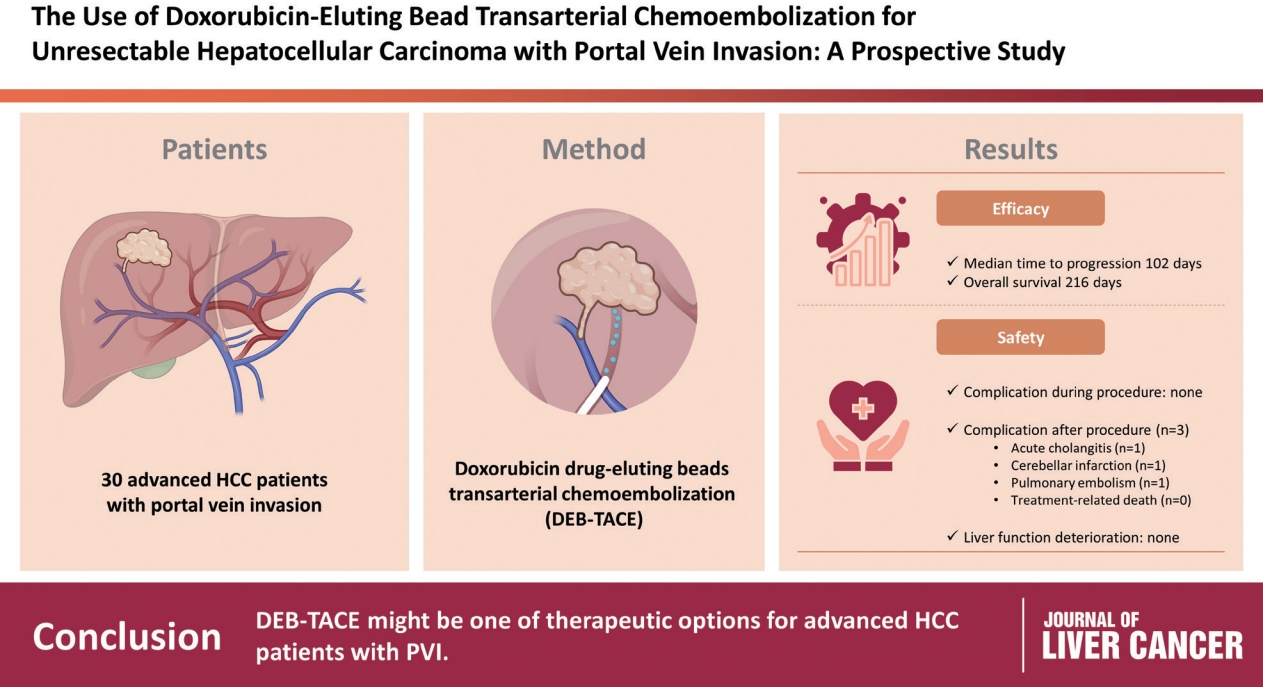
To evaluate the applicability of transarterial chemoembolization (TACE) treatment with doxorubicin drug-eluting beads (DEBs) in advanced hepatocellular carcinoma (HCC) patients with portal vein invasion (PVI).
Methods
This prospective study was approved by the institutional review board and informed consent was obtained from all participants. A total of 30 HCC patients with PVI received DEB-TACE between 2015 and 2018. The following parameters were evaluated: complications during DEB-TACE, abdominal pain, fever, and laboratory outcomes, including liver function change. Overall survival (OS), time to progression (TTP), and adverse events were also analyzed and assessed.
Results
DEBs measuring 100–300 μm in diameter were loaded with doxorubicin (150 mg per procedure). There were no complications during DEB-TACE and no significant differences in the levels of prothrombin time, serum albumin, or total bilirubin at follow-up compared to baseline. The median TTP was 102 days (95% confidence interval [CI], 42–207 days) and the median OS was 216 days (95% CI, 160–336 days). Three patients (10%) had severe adverse reactions, including transient acute cholangitis (n=1), cerebellar infarction (n=1), and pulmonary embolism (n=1), but no treatment-related death occurred.
Conclusions
DEB-TACE may be a therapeutic option for advanced HCC patients with PVI.

Case Reports
- Hepatocellular Carcinoma with Segmental Portal Vein Invasion Exhibiting a Complete Response after Transarterial Radioembolization
- Jun Sik Yoon, Su Jong Yu, Yun Bin Lee, Eun Ju Cho, Jeong-Hoon Lee, Yoon Jun Kim, Jung-Hwan Yoon
- J Liver Cancer. 2019;19(2):159-164. Published online September 30, 2019
- DOI: https://doi.org/10.17998/jlc.19.2.159

- 5,327 Views
- 73 Downloads
-
 Abstract
Abstract
 PDF
PDF - The treatment options available for patients with hepatocellular carcinoma (HCC) with portal vein invasion (PVI) include sorafenib, transarterial radioembolization (TARE), radiation therapy (RT), transarterial chemoembolization with RT, and proton beam irradiation. Herein, we present a case of HCC with segmental PVI that was managed via TARE. The patient had a 4 cm HCC that invaded the segment VIII portal vein branch without extrahepatic spread. Liver function was Child-Pugh grade A, and performance status was good. TARE was performed without any adverse events, and a radiological complete response (CR) was achieved. Thereafter, the patient was followed-up every 3-6 months without any further treatment, and the CR was maintained for >3 years. Therefore, TARE may be a useful alternative therapeutic option for patients with HCC exhibiting segmental PVI.

- A Case of Concurrent Chemoradiation Therapy for Locally Advanced Hepatocellular Carcinoma with Portal Vein Thrombosis
- Tae Young Yang, Suk Pyo Shin, Joo Ho Lee, Yun Bin Lee, Hana Park, Seong Gyu Hwang, Kyu Sung Rim
- J Liver Cancer. 2015;15(1):52-56. Published online March 31, 2015
- DOI: https://doi.org/10.17998/jlc.15.1.52
- 1,133 Views
- 7 Downloads
-
 Abstract
Abstract
 PDF
PDF - Patients with advanced hepatocellular carcinoma (HCC) with portal vein thrombosis (PVT) have an extremely poor prognosis. Although the Barcelona Clinic Liver Cancer guideline recommends sorafenib in advanced HCC with PVT, which has provided survival benefits of 2 or 3 months compared to the placebo group, many liver cancer centers in Asia still select multimodality approaches including transarterial chemoembolization, radiofrequency ablation, radiation therapy (RT) as well as systemic/intra-arterial chemotherapy. Recently advanced RT technologies have shown potential to improve survival without severe radiationrelated toxicity. For locally advanced HCC patients with PVT, concurrent chemoradiotherapy (CCRT) has been applied as a loco-regional treatment and provides potential cures. We herein report our recent experience of a patient accompanying large HCC with PVT who successfully undergone CCRT followed by hepatic arterial infusion chemotherapy.


 E-submission
E-submission THE KOREAN LIVER CANCER ASSOCIATION
THE KOREAN LIVER CANCER ASSOCIATION
 First
First Prev
Prev



 Follow JLC on Twitter
Follow JLC on Twitter